COVID-19 Vaccine Distribution
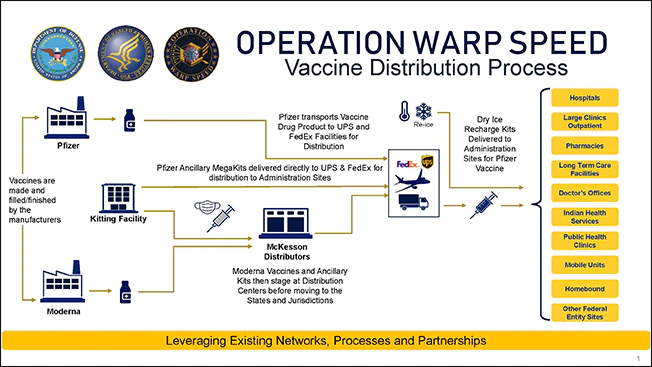
The COVID-19 vaccine distribution process leverages existing networks, processes and partnerships to make vaccines available across America as quickly and safely as possible. Each week, as doses are released by companies for distribution, planes and trucks transport the vaccine to states and jurisdictions across the country.
The federal government, through Operation Warp Speed, has been working since the pandemic started to develop, manufacture, and distribute COVID-19 vaccines. Safety is our top priority as we work to make coronavirus disease 2019 (COVID-19) vaccines available as soon as possible.
Overview of Vaccine Distribution Process
Authorization
On December 11, 2020, the U.S. Food and Drug Administration (FDA) issued the first emergency use authorization (EUA) for use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 16 years and older for the prevention of COVID-19.
On December 18, 2020, FDA issued the second EUA for use of the Moderna COVID-19 vaccine in persons aged 18 years and older for the prevention of COVID-19.
Prioritization
The Centers for Disease Control and Prevention (CDC) recommends the initial phase of the COVID-19 vaccination program be offered to healthcare personnel and residents of long-term care facilities. Governors and jurisdictions will ultimately decide who will receive the vaccines.
- Learn more about how CDC is making vaccine recommendations
- Read ACIP's recommendation for use of the Pfizer-BioNTech COVID-19 Vaccine
- Read ACIP's recommendation for use of the Moderna COVID-19 Vaccine
Allocation
The dataset lists the allocations of doses made available for states and jurisdictions to order against. Weekly allocations are provided to states on Tuesdays; after doses are ordered by states, shipments begin the following Monday. The entire order may not arrive in one shipment or on one day, but over the course of the week; delivery sites are notified by the private shipping partners. Shipments of an FDA-authorized safe and effective COVID-19 vaccine continue to arrive at sites across America. Vaccinations began on December 14, 2020.
- See the allocations for the Pfizer-BioNTech COVID-19 Vaccine by jurisdiction
- See the allocations for the Moderna COVID-19 Vaccine by jurisdiction
Distribution
Distribution of the vaccine began 24 hours after the EUA, with the first deliveries and vaccinations occurring on Monday, December 14, 2020. Shipments of an FDA-authorized safe and effective COVID-19 vaccine are arriving at sites across America. Vaccinations began on December 14, 2020.